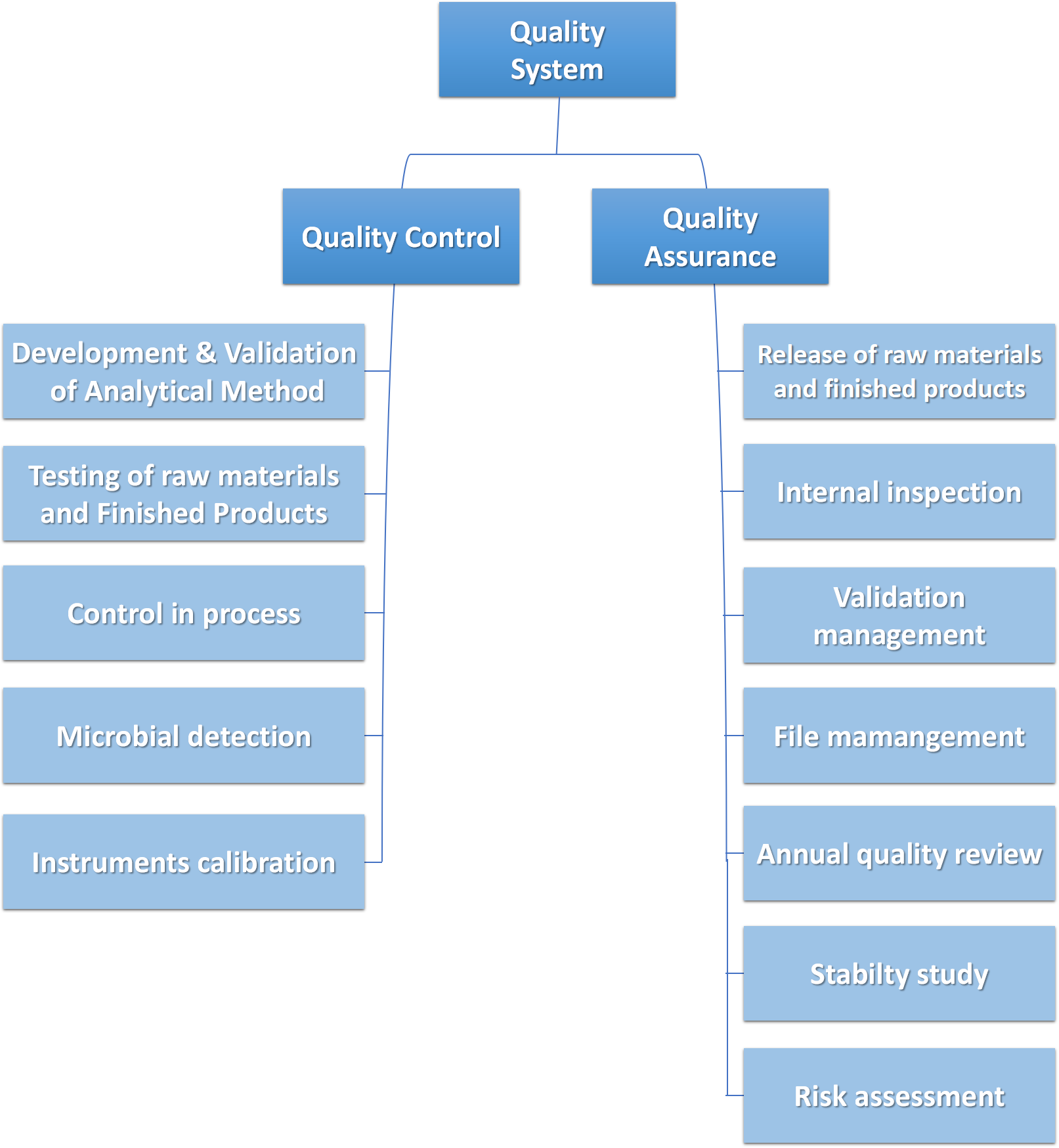
Wisdom quality control (QC) team ensure our products and services meet the customers' specific requirements and characteristics. Strictly follow the ICH guidelines, we exam and test the quality of the whole process of our products and services using the state-of-art instrument and labs. The QC team provide the reliable data to the quality assurance (QA) team for the release of materials and actions.
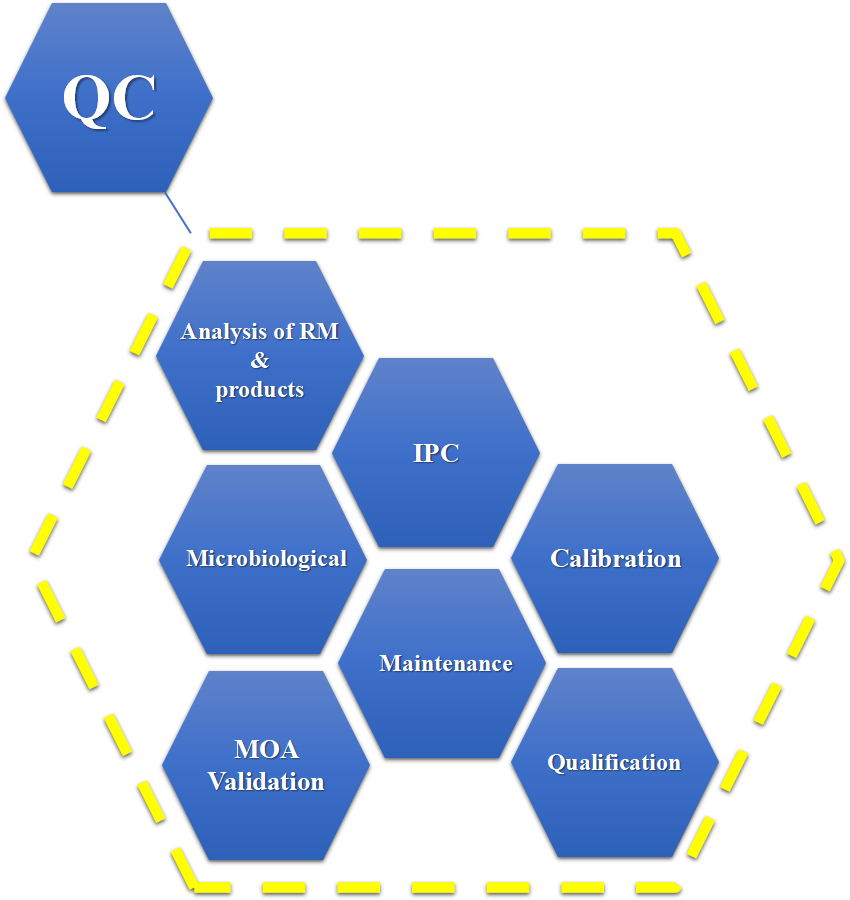
Our QC laboratories equipped with 6 platforms:
(NMR(Bruker 600 MHz), HPLC (with VWD/DAD/CAD/ELSD), GCs (with Headspace), Dionex ICS 5000+, UHPLC Ultimate 3000)
(TripleTOF(ABSciex 5600), GCMS/MS(Shimadzu TQ8040), GCMS(Shimadzu), LCMS(Shimadzu), Dionex ICS 5000+/2000+
(Malvern Mastersizer, Olympus CX31)
(ICP-MS, Microwave Digestion System, Dionex ICS 5000+)
(Class A bench for Positive Testing, Class A bench for Micro Testing)
We are equipped with Data Integrity System (Labsolution CS+). All computers are validated and OOS/OOT/deviation investigation system are well in place.
Our QC Laboratories are staffed by a team of highly trained and skilled chemists.
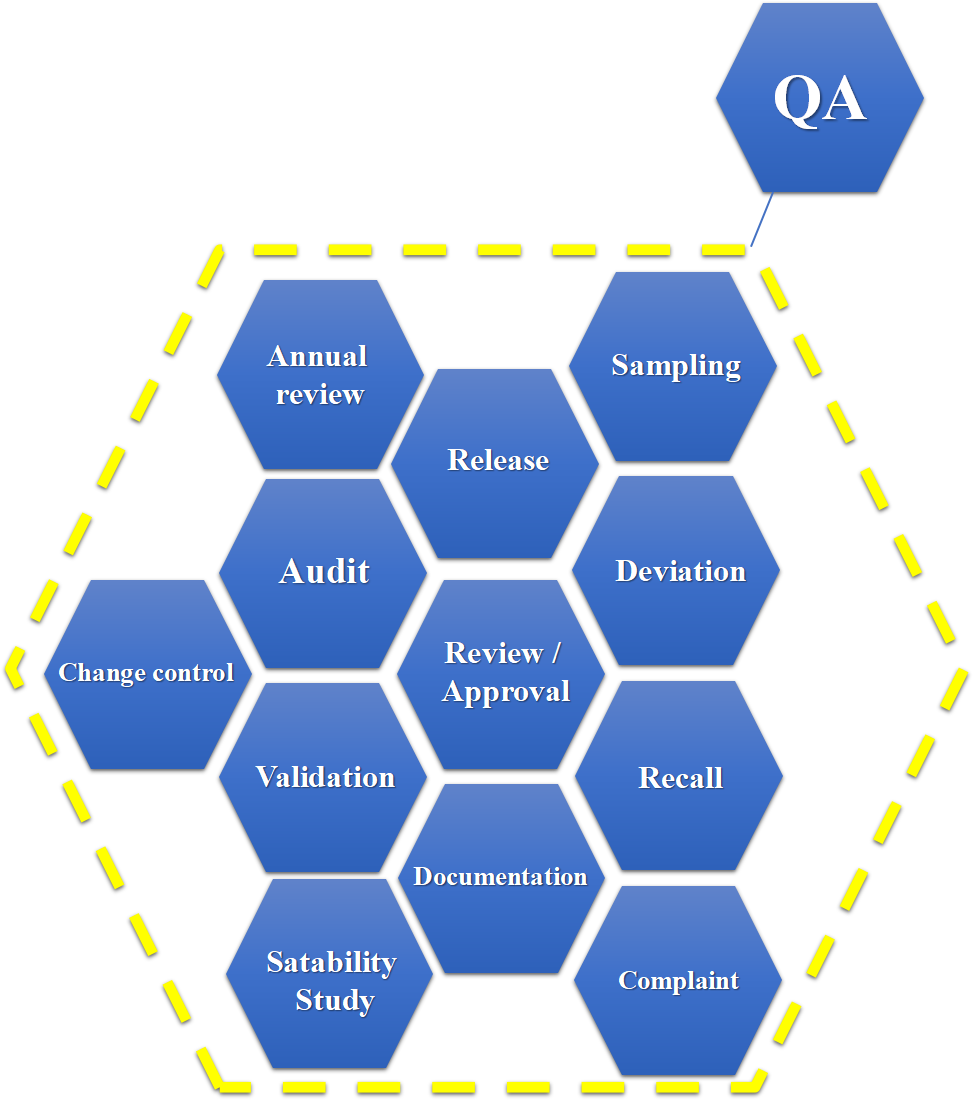
Wisdom Quality Assurance team participate, monitor, and manage the whole process of operations to develop and produce active pharmaceutical ingredients according to the high-quality standards defined by the current Good Manufacturing Practices, and the International Convention for Harmonization (ICH) Q7/Q8/Q9/Q10/Q11 guidelines.
To provide service to the worldwide regulated pharmaceutical markets, Wisdom has been frequently audited and qualified by global leading pharmaceutical companies and certified by FDA, EDQM, PMDA, SFDA, and MOA. The latest successful FDA inspection was in March 2018.



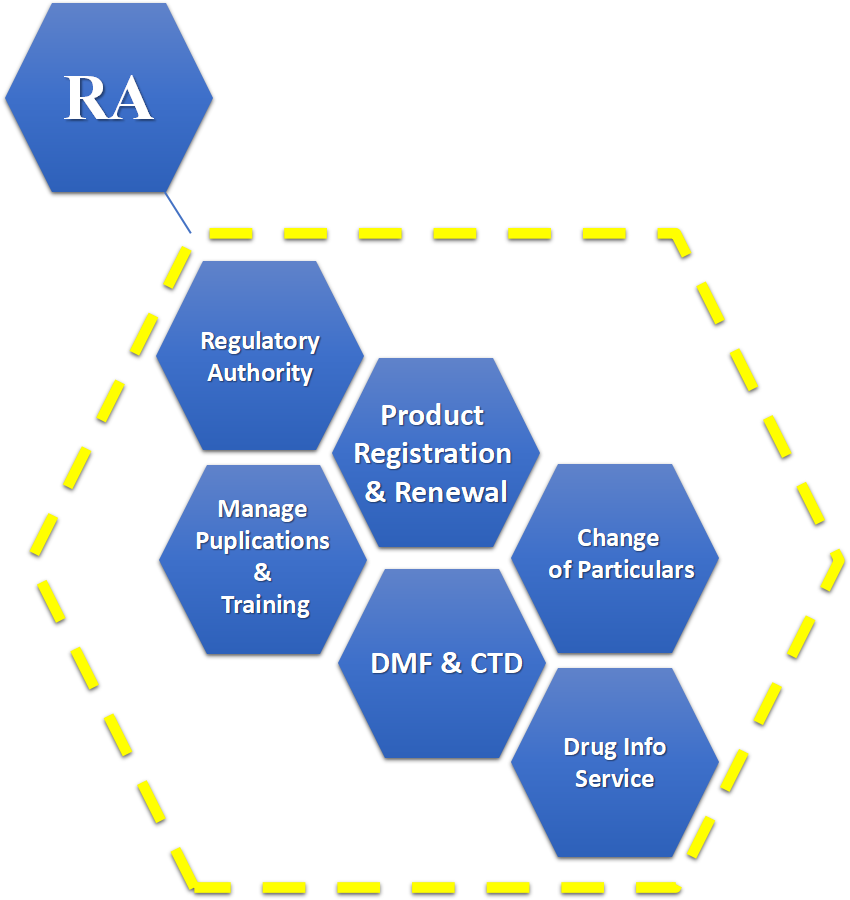
Wisdom regulatory department is responsible for communication with the regulatory authorities. We ensure the compliance with the latest regulatory requirements. We apply the latest regulation to our cGMP system.
Our regulatory team participates in the project management from the very beginning. We consider and include all regulatory elements in the project development, quality research, manufacturing, and preparation of documents.
We are experienced in DMF submission in CTD format or the country-specific requirement. We also support our customers for regulatory submission.

Copyright © 2017.Wisdom Pharmaceutical Co., Ltd. All rights reserved.www.wisdompharma.com